Brenda Blacklock, Ph.D.
Teaching Professor, Chemistry & Chemical Biology

Teaching Professor, Chemistry & Chemical Biology
Sphingolipids are a structurally diverse class of lipids found in all eukaryotes and several bacteria and are emerging as a particularly rich source of bioactive molecules. Functions of sphingolipids range from structural roles to signal transduction mediators that affect the regulation of cancer cell growth, differentiation, senescence, apoptosis, proliferation, inflammation, and cell motility. The number of molecular species of sphingolipids found in cells is astounding with diversification at three different positions within the sphingolipid molecule (the long-chain base, the long or very long chain acyl group and the head group). This combinatorial-like diversification suggests that a specific sphingolipid (that is, one with a defined long chain base, N-linked acyl chain and head group) may be required for each of the diverse functions of the class of sphingolipids.
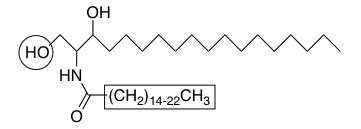
Ceramides are derived from the linkage of palmitoyl-CoA with serine followed by N-acylation with C16 to C24 fatty acids (boxed). More complex sphingolipids are produced by the addition of head groups at the C1 hydroxyl position (circled).
We are interested in the metabolic origins of the long and very long chain fatty acids (VLCFA) incorporated into biologically active lipids such as ceramides and other sphingolipids.
VLCFA (very long chain fatty acids) are unusual fatty acids with more than 18 carbon atoms that are incorporated into a variety of eukaryotic lipids including membrane sphingolipids and glycerolipids, seed storage oils and surface waxes in plants and eicosanoids, such as leukotrienes and prostaglandins in mammals. VLCFA are produced by the elongation of 16 and 18 carbon-long fatty acids made by the fatty acid synthase pathway. Their metabolism is best understood in plants where they are produced by a membrane-bound fatty acid elongase complex. Flux through the elongation pathway is controlled by an initial condensation step catalyzed by a 3-ketoacyl-CoA synthase (KCS).
Yeast and other organisms as widely ranging as microalgae, moss, fungi, worms and mammals appear to use a different fatty acid elongation pathway involving gene products called ELOs (for fatty acid elongation). Although studies have shown a role for ELOs in the production of 3-ketoacyl-CoAs, the direct product of classical fatty acid condensing enzymes, evidence that ELOs, themselves, are condensing enzymes is lacking. Many organisms with the ELO-based elongation pathway do not appear to possess KCS-like genes. ELOs may fulfill the role of the condensing enzyme in these organisms or an unknown condensing enzyme may be present. A role for ELO homologs in cancer is suggested by the overexpression of the human ELOVL family in prostate cancer.
In plants, the sequential elongation of plastid-derived fatty acids is accomplished by four distinct polypeptides catalyzing condensation, reduction and dehydration reactions. The function of ELOs as alternate condensing enzymes or KCS analogs is the primary goal of our research.
We are interested in uncovering the complementary and contrasting roles of the alternative elongation pathways and the function of sphingolipids that incorporate the fatty acid products of these pathways in signal transduction/developmental processes. Our approach to these questions uses an interesting organism that has been developed as a model for plant and animal cell motility, signal transduction, and development; Dictyostelium discoideum. D. discoideum is a social amoeba or cellular slime mold that lives as single vegetative cells when food (bacteria) are available and, upon starvation, preserves the colony through a developmental program that culminates in sporulation. Interestingly, both KCS and ELO genes are present in the D. discoideum genome and therefore, this organism represents an opportunity to dissect out the roles of KCSs and ELOs in fatty acid elongation. The accessible genetics, the unusual developmental profile and the ease of culture and biochemical characterization in the D. discoideum system, will be invaluable in the investigation of the physiological function of ELOs and KCSs.
Hernandez-Buquer, S. and Blacklock, B.J. Site-directed mutagenesis of a fatty acid elongase ELO-like condensing enzyme. (2013) FEBS Lett., 587, 3837-3842.
Yamasaki, Y., Koehler, G., Blacklock, B.J., and Randall, S.K. Dehydrin expression in soybean. (2013)Plant Physiol. Biochem., 70, 213-220.
Blazer-Yost, B.L, Blacklock, B.J., Flaig, S., Bacallao, R.L., and Gattone, V.H. Lysophosphatidic acid is a modulator of cyst growth in autosomal dominant polycystic kidney disease. (2011) Cell. Physiol. Biochem., 28, 1255-1264.
Blacklock, B.J., Scheffler, B.E., Shepard, M.R., Jayasuriya, N., Minto, R.E. Functional diversity in fungal fatty acid synthesis. The first acetylenase from the Pacific Golden Chanterelle, Cantharellus formosus. (2010) J. Biol. Chem., 285, 28442-28449.
Minto, R.E., Blacklock, B.J., Younus, H., Pratt, A. Atypical biosynthetic properties of a ∆12/ν+3 desaturase from the model Basidiomycete, Phanerochaete chrysosporium. (2009) Appl. Environ. Microbiol. 75, 1156-1164.
Blacklock, B.J., Kelley, D., and Patel, S. A fatty acid elongase ELO with novel activity from Dictyostelium discoideum. (2008) Biochem. Biophys. Res. Comm. 374, 226-230.
R. E. Minto and B. J. Blacklock "Biosynthesis and function of polyacetylenes and allied natural products" Prog. Lipid Res. 2008, 47, in press.
B. J. Blacklock and J. G. Jaworski "Substrate specificity of Arabidopsis 3-ketoacyl-CoA synthases" Biochem. Biophys. Res. Commun. 2006, 346, 583-590.
H. Moon, G. Chowrira, O. Rowland, B. J. Blacklock, M. A. Smith and L. Kunst "A root-specific condensing enzyme from Lesquerella fendleri that elongates very-long-chain saturated fatty acids" Plant Mol. Biol. 2004, 56, 917-927.
B. J. Blacklock and J. G. Jaworski "Studies into factors contributing to substrate specificity of membrane-bound 3-ketoacyl CoA synthases" Eur. J. Biochem. 2002, 269, 4789-4798.
J. G. Jaworski and B. J. Blacklock "Fatty Acid Elongase 3-Ketoacyl CoA Synthase Polypeptides" US patent 6, 713, 664 , World patent granted.
C. A. Parent, B. J. Blacklock, W. M. Froehlich, D. B. Murphy and P. N. Devreotes "G protein signaling events are activated at the leading edge of chemotactic cells" Cell 1998, 95, 81-91.